THERMAL STUDIES OF CHLORAL HYDRATE
THERMAL STUDIES OF CHLORAL HYDRATE
Arun G. Bhoi
Arun’s Institute of Forensic Sciences, Research and Education, Pune – 411028, State of Maharashtra, (India).
Email : arun.bhoi@gmail.com
(e-J. Foren. Crime Inv. 2012, 5, 1. Art. 1, 1st Jan. 2012)
ABSTRACT
Cases of liquor tragedies occur worldwide. This is because the consumption of illicit liquors which are either prepared and sold by adding chloral hydrate or it gets contaminated with the poisonous material like methyl alcohol. It is covered and penalized in Maharashtra (India) under Bombay Prohibition Act – 1949. The chloral hydrate induces the hypnotic effect and the latter causes blindness and/or death. Therefore, the thermal analysis of the chloral hydrate is carried out and its TGA¸ DTG and DTA properties are explored in this work. The kinetic parameters reaction order and activation energy are calculated using ‘Coats and Redfern’ equation.
KEY WORDS
Illicit liquor, chloral hydrate, hypnotic, thermal analysis, TGA, DTG, DTA, reaction order, activation energy, Coats – Redfern equation.
INTRODUCTION
Chloral hydrate 2,2,2 – trichloroethane 1,1 – diol is a sedative and hypnotic material. It is illegally added to the alcoholic beverages and its abuse leads to addiction (1). It is famous as “Knockout Drug”. In gastrointestinal tract chloral hydrate gets oxidized to tri-chloroacetic acid and partly reduced to trichloroethanol. The latter is responsible for hypnotic action (2). Being a sedative it is widely used in medicinal preparations and sold in the form of capsules, liquid formulations. It is, also, used as supposatory (3), rubefacient (2) and on treatment of asthma. Forensic scientist has to examine variety of samples from medico-legal cases comprising chloral hydrate when submitted for analysis by police / custom officers. Different methods are reported in the literature for analyzing chloral hydrate and its preparations such as ‘Fujiwara Test’ (4), spectrophotometry (5), IR spectrophotometry (6,7) and gas chromatography (8, 9). Least attention has been paid to its thermal studies and hence chosen to study TGA, DTG and DTA properties.
EXPERIMENTAL
Equipments
Thermal properties of chloral hydrate are studied by using TGA and DTA units developed and installed in the Department of Chemistry, University of Pune, Maharashtra, India.
Reagents
Chloral hydrate used for the experiment was of the Analytical Reagent Grade.
Procedure
The following specifications were used for the TGA (Thermogravimetric Analysis), DTG (Differential Thermogravimetry) and DTA (Differential Thermal Analysis) studies:
- Sample Size : 100 mg
- Sample Holder : Quartz Cups
- Temperature Range for Study : Room Temp. to 800°C
- Rate of Heating : 3-4 °C / min.
- Thermocouple Used : Chromel – alumel.
- Atmosphere : Static Air.
RESULTS AND DISCUSSION
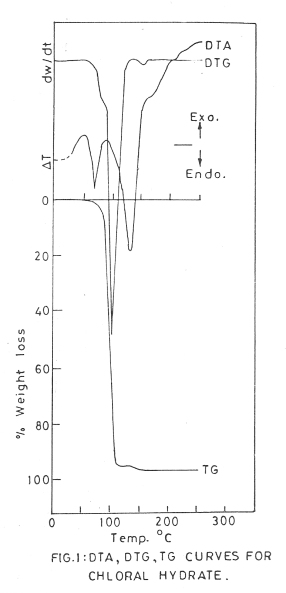
TGA, DTG and DTA studies of chloral hydrate, a monoclinic, perfect 001 crystal explains the thermal behavior of poly-halogen compounds. On TG curve slow rate of loss is seen in the range of 60° – 90°C. It can be attributed to the crystalline transition and vaporization of chloral hydrate. Maximum loss on TG curve is seen in the range of 90° – 110°C (Fig. 1). It could be attributed to the decomposition of chloral hydrate to tri-chloro-acetaldehyde and water and its simultaneous vaporization.
Cl3C.HC(OH)2 ———–> 90° – 110°C ———–> Cl3CHO + H2O
The further loss in the range of 110°C to 150°C could be attributed to the subsequent decomposition of choral resulting in the release of chloro compounds / gases and CO / CO2
The corresponding endothermic peaks for decomposition are also, noticed on DTG curve. The steep portion on ‘TG’ curve and ‘DTG’ peak corresponds to maximum slope.
The kinetic parameters, reaction order ( n ) and activation energy (Ea ) are deduced using ‘Coats and Redfern’ equation. These are n = 0.750 and Ea = 133.1 kj/mole.
The DTA studies are equally informative. The earlier endothermic peak is due to liquefaction, vaporization and decomposition of chloral hydrate and partly due to vapourization of products and helps to understand the decomposition reaction. The prominent endothermic peak could depict the decomposition of chloral and evolution of gases formed. The residual carbon gets converted into CO/CO2 in the temperature range 730° – 750°C and the loss is 100%.
CONCLUSION
The results are reproducible, however, negligible differences are noticed from sample to sample as the quality of material does play the role, here. Thus, thermal analytical aid proves to be a good tool for the analysts and forensic experts in determining and estimating the chloral hydrate in variety of samples.
ACKNOLEDGEMENT
Author’s thanks are due to Dr. G. N. Natu, Prof. S. B. Kulkarni of Department of Chemistry, University of Pune, Maharashtra, India and Director, Directorate of Forensic Science Laboratory, State of Maharashtra, India. The author’s thanks are also, due to ITAS, Mumbai, Maharashtra (India).
REFERENCES
- The Merck Index : An Encyclopedia of Chemicals and Drugs. Ninth Edition, Merck and Co., Inc. Rahway, N. J., U.S.A., 1976, p- 260.
- Satoskar R. S. and Bhandarkar S. D., Pharmacology and Pharmacotherapeutics, Eleventh Edition, Popular Prakashan Bombay, 1988, p- 87.
- Alfred Goodman, Gilman L. S. and Alfred Gilman, The Pharmacological Basis of Therapeutics, Eighth Edition, Pergamon Press, New York, 1990, p- 365.
- Fujiwara K., Sber. Abh. Naturf. Ges. Rostock, 1916, 6, p- 33.
- Mahal H. S., Barve V. P. and Kamat S. S., Analyst, 1972, 97, p- 877-879.
- Clrke E.G.C., Isolation and Identification of Drugs, The Pharmaceutical Press, London, 1974, p- 245.
- DMS Working Atlas of Infra-red Spectroscopy, Butterworths and Co. (Pub.) Ltd., London, 1972.
- Amin D., Al-Asley M. S., Talanta, 1981, 28(12), p- 955-956.
- Archer A. W. and Haugas E. A., J. Pharma. Pharmae., 1960, 12, p- 754.
- Coats A. W. and Redfern J. P., Nature, 1964, 201, p- 68.