ESTIMATION OF HERBICIDE 2,4-D IN COMMERCIAL PREPARATIONS USING SPECTROPHOTOMETRY.
ESTIMATION OF HERBICIDE 2,4-D IN COMMERCIAL PREPARATIONS USING SPECTROPHOTOMETRY.
Arun G. Bhoi
Arun’s Institute of Forensic Sciences, Research and Education, Pune -411 028 (India).
Email : arun.bhoi@gmail.com
(e-J. Foren. Crime Inv. 2011, 4, 1. Art. 1, 11th Nov. 2011)
ABSTRACT
A spectrophotometric method is explained for the quantitative determination of 2,4-D (2,4-Dichlorophenoxyacetic acid) present in the commercial preparations. 2,4-D was quantified at λ-max 287 nm and linear function of concentration was observed in the range of 8 μg/ml to 20 μg/ml. The correlation coefficient was found to be o.9985. The presence of 2,4- D was confirmed by TLC using solvent system methyl alcohol : ammonia (100 :1.5 v/v) and Dragendorff’s reagent. It showed brown spot at Rf – value o.65.
KEY WORDS
2,4-D, herbicide, weedicide, spectrophotometric determination, TLC, Draggendorff’s reagent.
INTRODUCTION
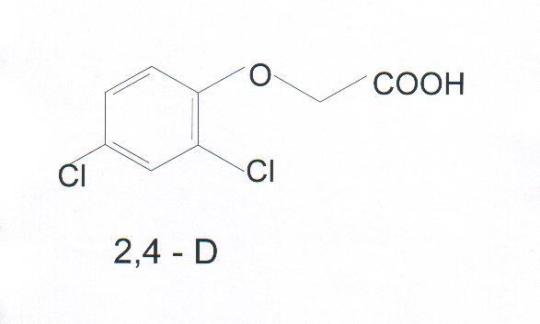
2,4- Dichlorophenoxyacetic acid and its sodium salts, as also, amine salts are commonly used as weedicides. These are, also, preferred as growth regulators, powerful and systemic herbicides. 2,4-D acid and its salts are selective herbicides used to control broad leaf weeds in farms of orchids, tea, sugarcane because of their total water solubility and systemic post emergent property. Its poisoning aspects are studied by Fianagan et. al. (1) Rivers et. al. studied the gas chromatographic detection of 2,4-D (2). Curry (3) studied the poisoning aspects through autopsy. In this work 2,4-D acid is detected using thin-layer chromatography and it is estimated by application of spectrophotometrytechnique.
Reagents
All reagents and solvents used were of analytical reagent grade. Distilled water and 95% ethyl alcohol were used throughout the experiment, wherever necessary.
Standard 2,4-D solutions:
The Commercial Grade 2,4-D preparation was procured from the market was used for the experimental studies. Standard solutions containing 8, 10, 12, 14, 16, 18 and 20 µg of 2,4-D per milliliter were prepared using requisite quantities of 2,4-D in 95% ethyl alcohol, in separate measuring tubes.
PROCEDURE
Thin Layer Chromatography
The glass plates (20 cm x 20 cm) were coated with slurry of silica Gel G with water (1:2) to a thickness of 0.25 mm. The plates were air dried at room temperature and then activated at 1050 C – 1100 C at least for half an hour. Std. Microgram quantities of 2,4 – D solution were spotted on the activated TLC plates. The plates were developed in a chamber (saturated) containing solvent system methyl alcohol : ammonia (100 :1.5 v/v). After the solvent front had travelled 10 cm distance from the original spotting line, the plates were taken out from the chamber and dried in air. The spots were visualized by spraying the TLC plate with Draggendorff’s reagent.
Spectrophotometric Determination:
2,4-D was quantitatively determined using spectrophotometric device Specord 200 – 1.8 – 0502. The absorbances of every standard 2,4-D solutions were measured at λ-max : 287 ± 2 nm in the UV range 200 – 340 nm using pair of matching quartz cells. 95% ethyl alcohol was used as a reference material. The respective absorbances are tabulated as below in Table – 1.
Table – 1: Table showing concentrations of standard 2,4-D solutions and its respective absorbances at λ-max : 287 ± 2 nm.
|
Sr. NO. |
Conc. of 2,4-D μg / ml |
Absorbance at λ-max : 287 ± 2 nm |
|
1 |
8 |
0.0296 |
|
2 |
10 |
0.0419 |
|
3 |
12 |
0.0634 |
|
4 |
14 |
0.0823 |
|
5 |
16 |
0.1025 |
|
6 |
18 |
0.1163 |
|
7 |
20 |
0.1365 |
RESULTS & DISCUSSION :
The TLC plates spotted with standard 2,4-D, eluted using above mentioned solvent system were air dried and sprayed with routinely used Draggendorff’s reagent. It showed the presence of 2,4-D as a brown spot at Rf – value 0. 65.
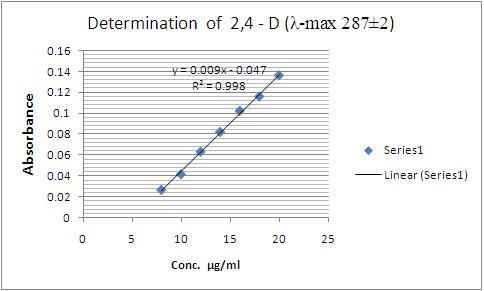
The absorbances of standard 2,4-D solutions measured at λ-max 287±2 nm with corresponding concentrations of 2,4-D are recorded in Table – 1. The calibration curve of these absorbances versus respective concentrations of 2,4-D procured is as shown below in Fig. 1.
Figure – 1 : Calibration curve for the determination of 2,4-D, Conc. of std. 2,4-D (μg/ml) vs. Absorbance measured at λ-max 287±2 nm
Beer’s law is obeyed good in the range of 8 μg to 20 μg per ml. The calibration equation found is “ Y = 0.009x – 0.047” and “correlation coefficient = 0.998”. Detection Limit was < 1 μg/ml. Diluents in the commercial preparations did not interfere in the measurements. This method can also be applied to the forensic toxicological analyses, successfully.
Acknowledgements
The authors thanks are due to the Director, Directorate of Forensic Science Laboratories, Mumbai, M. S.(India) and Chitra S. Kamat, Regional Forensic Science Laboratory, Nashik, M. S., (India).
REFERENCES
1) Fianagan, R.J., Meredith, T.J., Ruprah, M.,Onyon, L.J. and Liddie, The lancet, 1990, Feb 24, 454-458.
2) Rivers, J.B., Yauger, Jr. W.L and Klemaner, H.W.; J. of chromatography, 1970, 50, 334-337.
3) Curry, A.S.; British Medical Journal, 1962, 10,687-688.