Spectrophotometric Determination of Pyridine in Denatured Spirits
Arun G. Bhoi
Arun’s Institute of Forensic Sciences, Research and Education, Pune – 411028, State of Maharashtra, (India).
Email : arun.bhoi@gmail.com
(e-J. Foren. Crime Inv. 2011, 1, 1. Art. 3, 26th Jan. 2011)
ABSTRACT
The rectified spirit (ethyl alcohol) is intentionally denatured using denaturants like caoutchoucine, pyridine, crystal violet, denatonium saccharide, some aldehydes and petroleum hydrocarbons for specific use or to avoid its misuse. A spectroscopic method is reported here for the determination of pyridine in denatured spirit samples (DNS). The spirit samples containing pyridine are allowed to react with tri-chloroacetic acid in the presence of sodium hydroxide at 85˚c temperature for 75 minutes. The absorbance of the crimson colour produced is measured at λ-max 362 ± 1 nm. Bear’s law is found in the range of 275μg to 1472 μg. The other denaturants present in the denatured spirit samples do not interfere.
KEY WORDS : pyridine, denatured spirit, denaturant, sodium hydroxide, tri-chloroacetic acid.
INTRODUCTION
Various miscible denaturants such as acetaldehyde, acetone, caoutchoucine, formaldehyde, acetaldehyde, crotonaldehyde, ethyl acetate, ether, methanol, chloroform, pyridine, petroleum hydrocarbon solvent etc. are added to rectified spirit to make the later unfit for human consumption. Usually 0.5% (v/v) of pyridine bases are added to rectified spirit to make it non-potable. Many of the methods like gas chromatography [1,2], spectrophotometry [3-6], titremetry [7] and thin-layer chromatography [8] are reported in the literature for determination of pyridine.
The spectrophotometric method using tri-chloroacetic acid in alkaline condition is described here for the determination of pyridine.
EXPERIMENTAL
Equipments :
A Shimadzu double beam ultra violet / visible spectrophotometer and a matching pair of quartz cells were used for the absorbance measurements.
Reagents :
All reagents used were of Analytical Reagent grade. Glass distilled water was used throughout the experiment. 95 % ethyl alcohol was used as and when required.
- Sodium hydroxide solution (10%) : 10 g of sodium hydroxide palettes were dissolved in small quantity of distilled water and made up to 100 ml with the same.
- Tri-chloroacetic acid solution (1%) : 1 g of tri-chloroacetic acid was dissolved in distilled water and made up to 100 ml.
- Standard Pyridine solution (1 %) : Pyridine ( 1 ml ) was dissolved in 95% of ethyl alcohol and was made up to 100 ml.
Procedure :
Exact quantities ( ml ) of standard pyridine solution such as 0.3, 0.6, 0.9, 1.2 and 1.5 ml equivalent to 294, 588, 883, 1177 and 1472 μg respectively, were taken in separate reaction/test tubes. Distilled water was added to each of these to make the total volume 3 ml. For blank 3 ml of distilled water was in one additional test tube. Then 2 ml of 10 % sodium hydroxide solution and 5 ml of 1 % tri-chloroacetic acid solution was added to each of these reaction tubes. The water bath was adjusted to 85˚c approx. and all the above reaction tubes were kept in the water bath for 75 min. The temperature of water bath was regulated to 85˚c during the reaction time. Afterwards the tubes were taken out and allowed to stand for half an hour. Then the absorbances of the crimson color developed were measured at λ-max 362 ± 1 nm using blank reagent as reference with the help of double beam Shimadzu ultraviolet – visible spectrophotometer and matching pair of quartz cells.
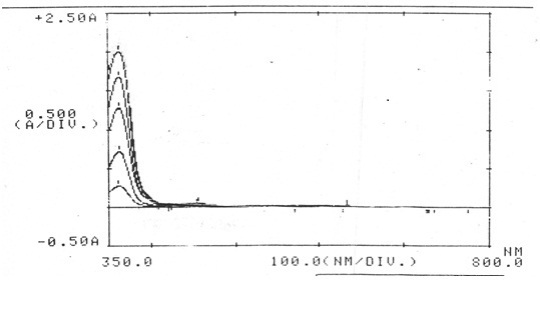
Figure 1 : Showing absorbance spectra of colour developed for Pyridine Standard 1 to Standard 5 (λ-max 362±1 nm).
TABLE 1 : Showing Concentrations of Standard Pyridine /10ml and its corresponding Absorbances
| StandardNo. | Concentration of Std. Pyridine per 10 ml | Absorbance (Av. of two)At λ-max 362 ±1 nm | |
| ml of 1% Std. Pyridine | Equivalent μg of Pyridine | ||
| 1 | 0.30 | 294 | 0.266 |
| 2 | 0.60 | 588 | 0.670 |
| 3 | 0.90 | 833 | 1.099 |
| 4 | 1.20 | 1177 | 1.594 |
| 5 | 1.50 | 1472 | 1.960 |
RESULTS AND DISCUSSION
Fig. 1 shows the absorbance spectra of the crimson color developed. The absorbances with corresponding concentrations of pyridine are recorded in Table – 1. The calibration curve of absorbance versus respective concentration of pyridine is shown in Fig. 2. Beer’s law is obeyed good in the range of 275 μg to 1472 μg per 10 ml. The calibration equation for this is “ Y = 0.001x – 0.158” and “correlation coefficient = 0.997”. Other denaturants such as acetone, aldehydes, methanol, chloroform, pyridine, ethylacetate, ether etc. did not interfere in the determination of the pyridine. Thus, this method is very much useful for the determination of pyridine in spirit (DNS). The requisite quantities of the DNS samples can be taken directly for the estimation of pyridine along with standards for colour development. By measuring absorbance of the colour developed at λ-max 362±1 nm, the corresponding concentration of pyridine can be found from calibration curve. Thus the method reported here can be used preferentially for the determination of pyridine in DNS and does not require any pre-treatment of the sample.
AKNOWLEDGEMENT
Author’s thanks are due to Director, Directorate of Forensic Science Laboratories, State of Maharashtra, India.
REFERENCES
- Habboush, A. E., Farroha, S. M. and Al-Bayat, R. I.; Chromatographia., 1978, 11, 662.
- Iskenderov, R. A., Ali-Zade, N. I., Bairamov, F. G. and Nagiev, T. M.; Zh. Anal. Khim., 1988, 43, 181 (Russ.).
- Zhadanov, B. V., Dobrakova, G. M. and Adamova, G. M.; Ref. Zh. Khim., 1977, 20, 19 GD.
- Rawat, J. P. and Bhattacharjee Priti; Indian J. Chem., Sect. A, 1976, 14, 544.
- Amlathe, S., Upadhyay, S. and Gupta, V. K.; Microchem. J., 1988, 37(2), 225.
- Ramchandran, K. N. and Gupta, V. K.; Microchem. J., 1991, 44 (3), 272.
- Indian Standard, Specification for Ordinary Spirit (Revised), Indian Standards Institution, New Delhi, 18:324, 1959, p.26.
- Land, E. and Lang, H. Z.; Analyt. Chem., 1976, 278, 368.

Figure 2 : Calibration curve for the determination of pyridine, Conc. of std. pyridine (μg/10ml) vs. Absorbance measured at λ-max 362±1 nm